An Unusual spessartine garnet
An unusual gem spessartine interests dr. bill hanneman and I
The garnet group is chemically and structurally complex and gemologists have for decades persisted in attempting to classify gem garnets based on their measurable physical and optical qualities, as opposed to the mineralogical approach of determination by chemical and structural analysis. Generally speaking, the gemological approach works quite well as there are relationships between the easily measurable physical and optical properties and the chemistry and crystal structure of the gem. However, the results predicted from the color, spectrum, refractive indices and specific gravity are not always accurate and this is the case for the gem spessartine garnet we are about to describe.
Nomenclature is, in general, a problem in mineralogy where some 4300 distinct minerals exist but where there are about 14,000 known mineral names! Yet, the chemical composition determined by one of the many applicable instrumental analytical techniques or wet chemical methods coupled with x-ray diffraction analysis for structural determination leaves no ambiguity when it comes to mineral description and identification. Every mineral is defined by its chemistry and structure.
Generally, the gem garnets have been well described and documented by the gemological community but there has been on-going refinement of how one defines various species and varieties based on the optical and physical properties of the gem that a gemologist might measure. For instance, when does a pyrope-almandine garnet become just a pyrope or just an almandine? In earlier days, industry pioneers like Robert Webster, the author of Gems; Basil Anderson, the author of Gem Testing; and Richard Liddicoat, the author of the Handbook of Gem Identification established rather arbitrary cut-off points for the definitions in terms of physical and optical properties. As time progressed it became obvious that a more definitive and sensible method was necessary to both define intermediate species and newly discovered garnet types that were popular enough to have been given varietal or trade names by the gemological community.

When John Dyer, a well known gem cutter, wanted to make sure he was selling an orange garnet by the correct name he sent me two rough samples for analysis. The R.I. of each sample was 1.808 (measured with a calibrated refractometer, higher RI fluid {1.83} and with a Na light source) and the S.G. 4.20, the spectroscope clearly displayed a Mn spectra and the inclusions were definitive for spessartine. So, there seemed to be no question about what to call the slightly reddish orange samples. Or was there? Instead of just spessartine, could the spessartine component be less than 70%? Could the sample fairly be called almandine-spessartine garnet? The Challenger gemological spectrometer showed vary faint iron lines as well. Just how much iron was in the sample?
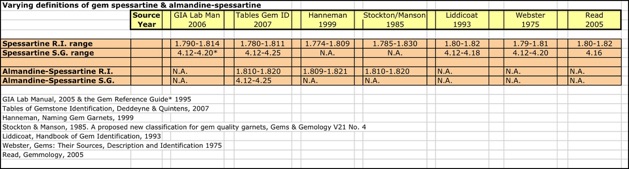
An R.I. of 1.808 and an S.G. of 4.20 would qualify the garnet sample as spessartine under almost all definitions, but according to Hanneman, it was right on the border of 30 mole % almandine. In other words, it was close to being called an almandine-spessartine.
My next step was to check one of the bibles of hard rock geology, the set of six volumes entitled Rock Forming Minerals by Deer, Howie and Zussman. The original edition dates back to the early 1960s but the latest edition was completed in 1997 and was reprinted in 2001 and the answers I was looking for are in Volume 1A, Orthosilicates. In 1957 a mineralogist named Sriramadas developed 8 ternary (e.g. triangular) diagrams using the five major garnet components of spessartine, almandine, pyrope, grossular and andradite, which allows the composition of the garnet to be found within the context of three components using the refractive index and the unit cell edge length.
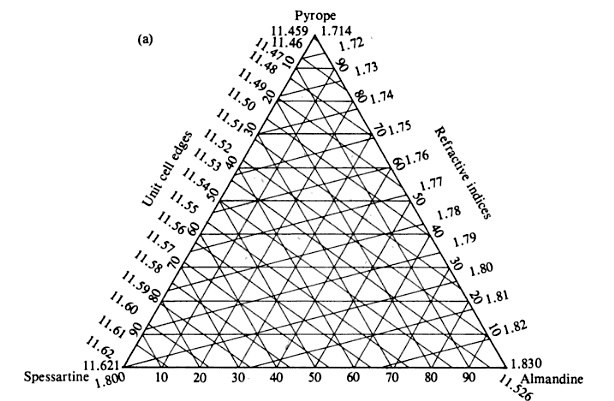
Plugging the 1.808 value into the diagram above translates to a garnet composed of approximately 70% spessartine and 30% almandine, right on the dividing line.
It seems clear that Hanneman relied on four of these ternary diagrams to construct his Rosetta stone of garnets as the R.I. correlations with composition appear identical to those derived from Sriramadas work based on the theoretical end-member composition work done by Skinner a year earlier. Hanneman added the important color data and determined the boundaries that directly relate to current nomenclature.
So where does that leave us with the Dyer spessartine? Since the literature leads us to believe that the specimen could be on the borderline, there is only one way to find out. Get the chemical composition by electron microprobe and the unit cell edge length by x-ray diffraction. That’s what I did.
The x-ray diffraction results gave a=11.594 Angstroms and there was notable fluorescence on the image plate from Cu-K-alpha radiation indicating the presence of some iron. Going back to the diagram above and adding the unit cell edge length we get a correlation with R.I. and chemistry of about 27-28% almandine and 71-72 mole % spessartine and perhaps 2% pyrope. Sounds pretty definitive. Yet the true chemical composition will tell the tale.
I used two different electron microprobes to analyze pieces of the same sample and the results are shown below as specimens 1 and 1A.
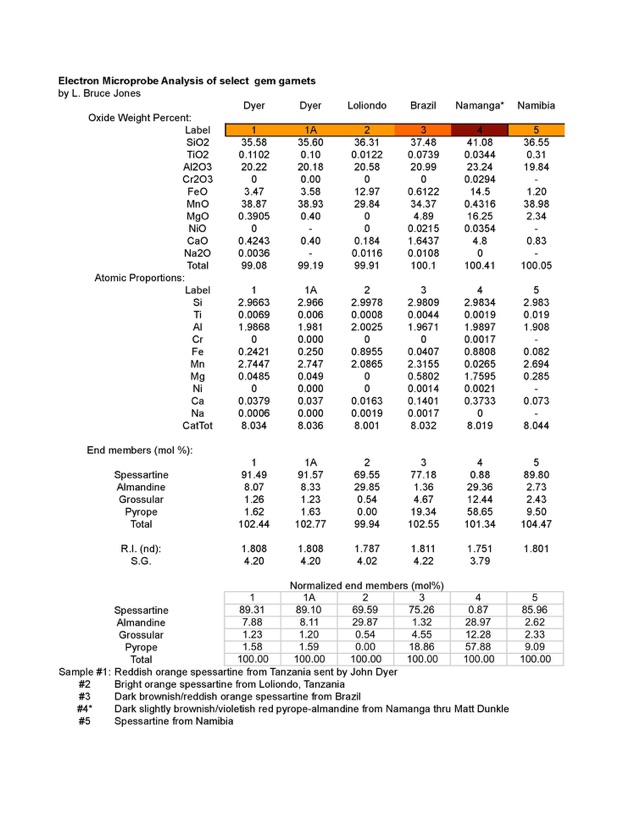
As it turns out, the Dyer specimen is a far cry from what was predicted. It is roughly 89% spessartine, 8% almandine and about 1.5 % pyrope and 1.2% grossular. As spessartine garnets go, it’s a relatively pure sample.
The results are a cautionary tale on using physical constants to extraplate chemistry in the complex garnet series of gems. Why does this problem exist specific to the Dyer spessartine? That’s a question for another day. Stay tuned...
